Some people who took a new schizophrenia drug for a year improved with only a few side effects, but many dropped out of the research, the company announced Thursday.
The results underscore the difficulties in treating schizophrenia, a severe mental illness that can cause people to hear voices, feel paranoid and withdraw from others. High dropout rates are typical in schizophrenia drug studies.
Finding a drug that works can be a long ordeal punctuated by crises and hospitalizations. Side effects of existing medications — weight gain, tremors, restlessness — cause some people to stop taking medicine and relapse.
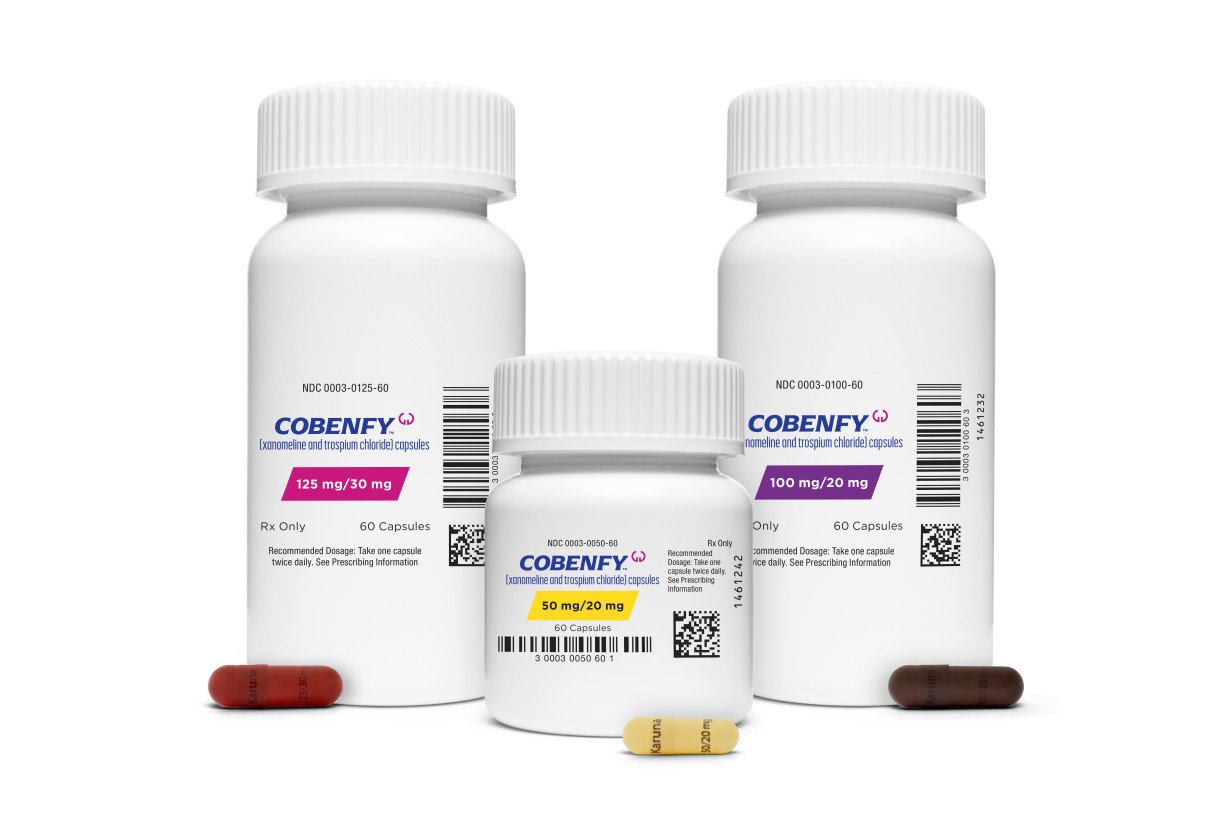
There's been great hope among doctors for Cobenfy, which was approved in September, because it acts in the brain differently than other schizophrenia drugs. Instead of blocking dopamine receptors, Cobenfy’s main ingredient, xanomeline, works on a different receptor that indirectly blocks dopamine release.
Cobenfy also contains trospium, which blocks some of the side effects. The most common are nausea, vomiting and indigestion. In contrast to the weight gain seen with other schizophrenia drugs, people lost a few pounds while taking Cobenfy, made by Bristol Myers Squibb.
Dr. John Krystal of Yale University has led research on other schizophrenia drugs but was not involved in the new studies. He noted that just 10% to 20% of participants in the new studies dropped out because of side effects.
“That is pretty good,” he said, noting that fewer or milder side effects could mean people will stay in treatment longer. That could mean fewer problems associated with untreated mental illness: substance use, homelessness and unemployment.
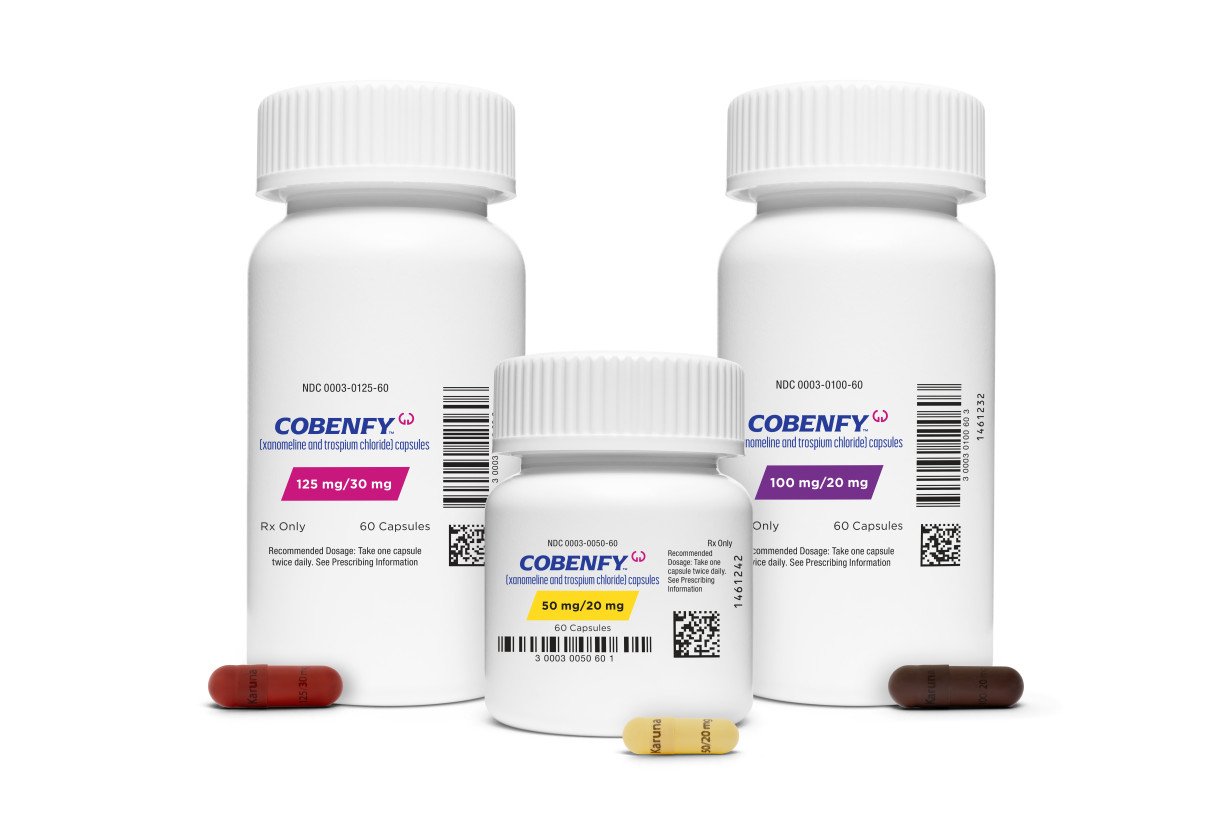
So why did some patients stick with treatment while others dropped out? Krystal said it will be important to understand more about that as doctors start prescribing the drug.
The Food and Drug Administration approved Cobenfy on the strength of two encouraging company-sponsored five-week trials and other safety data. The latest results announced Thursday at the Psych Congress meeting in Boston come from two longer studies, providing a fuller picture.
In one study, focused on severely ill patients, 78% dropped out, leaving only 35 people for the final analysis. In the other, focused on more stable people, 51% left the study, leaving 283 who took the drug for a year.
“It’s not any higher or any lower than what we typically see” in schizophrenia studies, said Dr. Greg Mattingly of Washington University School of Medicine in St. Louis. Mattingly is a consultant for Bristol Myers Squibb and a researcher on one of the studies.

In the more severely ill group, 69% of people had a meaningful improvement in their symptoms at the end of the year. In the other group, 30% saw a meaningful benefit.
Results of interviews with a sample of study participants conducted by an independent research team and shared by Bristol Myers Squibb showed the likelihood of continuing treatment. After six months, 36 said they would continue taking Cobenfy after the trial if given the option; 10 said they would not. Some participants said the drug reduced the voices while others said it didn't work for them.
The estimated yearly cost for Cobenfy is $22,500 compared to $540 for a generic antipsychotic. Krystal and others worry that insurers will require people to try cheaper drugs first before covering Cobenfy. Most patients’ out-of-pocket costs will be much lower, depending on insurance and other factors.
One cheaper generic called clozapine is widely considered one of the best treatments for schizophrenia, Krystal said. It is underused in the U.S. compared to some other countries because of a cumbersome blood testing program.
The FDA started the blood tests to watch for the risk of severe neutropenia, a rare side effect which can be fatal. But doctors and families have told the FDA that patients have relapsed when their clozapine was withheld or delayed because of the testing requirements.
Sally Littlefield, 29, of Alameda, California, said what works for her is a monthly injection of a long-acting antipsychotic medication. Littlefield, who has schizophrenia and bipolar disorder, wants to learn more about the experiences of people who've taken Cobenfy and not just from players with a financial stake.
Mindy Greiling of Roseville, Minnesota, wants to see data on how Cobenfy compares to clozapine, which works for her 47-year-old son, Jim. Weight gain was a problem for him, but since taking diabetes medication, he’s back to his normal weight, Greiling said.
Cobenfy “is getting a lot of ballyhoo, as any new drug does,” Greiling said. “It’s just a nonstarter for me unless it turns out that it’s better than clozapine.”
___
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group. The AP is solely responsible for all content.

 Greek government debt upgraded to investment grade, closing the door on a painful era
Greek government debt upgraded to investment grade, closing the door on a painful era
 West End beats Broadway in theatre revival. What's the secret?
West End beats Broadway in theatre revival. What's the secret?
 OpenAI and Musk agree to fast tracked trial over for-profit shift
OpenAI and Musk agree to fast tracked trial over for-profit shift
 Vatican switchboard nuns field growing calls about pope — but no, you can’t speak with him directly
Vatican switchboard nuns field growing calls about pope — but no, you can’t speak with him directly
 Zuby Ejiofor much more than just most improved in record-setting performance for No. 6 St. John's
Zuby Ejiofor much more than just most improved in record-setting performance for No. 6 St. John's
 Withers' lane violation adds improbable twist to UNC's failed comeback against No. 1 Duke in ACCs
Withers' lane violation adds improbable twist to UNC's failed comeback against No. 1 Duke in ACCs