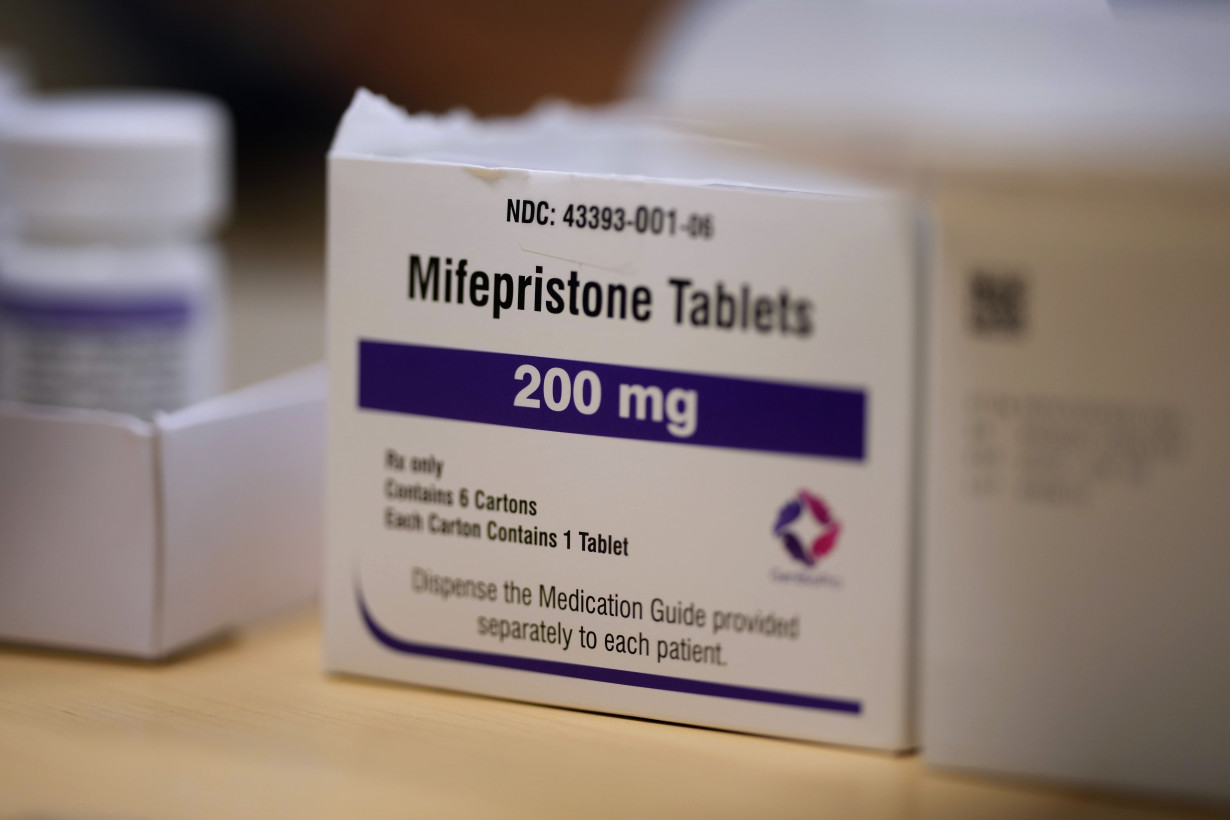
Many Americans wonder whether the pill used in most U.S. abortions will be restricted under the new Republican administration in Washington.
President Donald Trump’s pick to lead the Food and Drug Administration, Dr. Marty Makary, was confirmed by the Senate late Tuesday. In recent testimony before the Senate health committee, Makary wouldn’t commit to specific action on the pill, called mifepristone, despite prodding from both Republicans and Democrats.
Medical professionals call it “among the safest medications” ever approved by the FDA. But a Christian conservative group that sued the FDA over the drug says it has caused “tens of thousands” of “emergency complications."
Here’s what to know about the safety of mifepristone, which is typically used with misoprostol in medication abortions that make up close to two-thirds of abortions in the U.S.
What are the safety limits on use of the abortion pill?
The FDA approved mifepristone in 2000 as a safe and effective way to end early pregnancies. Currently, there are no in-person requirements and the pill can be sent through the mail.
That's not how the FDA first treated mifepristone, which on rare occasions can cause dangerous, excessive bleeding that requires emergency care. Strict safety limits were placed on who could prescribe and distribute it — only specially certified physicians and only as part of three mandatory in-person appointments with the patient getting the drug. The doctors also had to be capable of performing emergency surgery to stop excess bleeding and an abortion procedure if the drug didn’t end the pregnancy.
Over time, the FDA reaffirmed mifepristone’s safety and repeatedly eased restrictions.
How often are there serious problems?
Abortion opponents say the FDA’s 2021 decision allowing online prescribing and mail-order use of mifepristone resulted in many more “emergency complications.”
But that argument lumps together women experiencing a range of issues — from mifepristone not working to people who may simply have questions or concerns but don’t require medical care.
OB-GYNs say a tiny fraction of patients suffer “major” or “serious” adverse events after taking mifepristone.
A legal brief filed with the Supreme Court last year by a group of medical organizations including the American College of Obstetricians and Gynecologists says: “When used in medication abortion, major adverse events — significant infection, excessive blood loss, or hospitalization — occur in less than 0.32% of patients, according to a highly regarded study with more than 50,000 patients.”
The definition that scientists generally use for serious adverse events includes blood transfusions, major surgery, hospital admissions and death, said Ushma Upadhyay, one of the authors of that 2015 study.
The prescribing information included in the packaging for mifepristone tablets lists slightly different statistics for what it calls “serious adverse reactions.” It cites ranges for how frequently various complications occur: 0.03% to 0.5% for transfusion; 0.2% for sepsis and 0.04% to 0.6% for hospitalization related to medication abortions. The ranges reflect findings across various relevant studies, experts said.
Why do patients go to the emergency room?
Mifepristone’s labeling lists a complication that most medical groups don’t consider a serious or major adverse event: ER visits, which ranged from 2.9% to 4.6%. The current FDA label lists going to the ER as an option if patients experience prolonged heavy bleeding, severe abdominal pain or a sustained fever. But ER visits don’t always reflect big problems.
Some people may go there after a medication abortion because they want to be checked out or have questions but don’t have a doctor, said Upadhyay, a public health scientist at the University of California, San Francisco. Others, she said, “don’t want to go to their primary care provider about their abortion” because of stigma.
A study she co-authored in 2018 found that slightly more than half of patients who visited the ER because of abortions received only observational care.
How effective is the pill?
Mifepristone results in a completed abortion 97.4% of the time, according to U.S. studies cited in the FDA label.
But in 2.6% of cases, a surgical intervention is needed. And 0.7% of the time, the pregnancy continues.
That’s compared to a procedural abortion in a clinic, where the chance of the procedure failing to end a pregnancy “is extremely, extremely low,” probably less than 0.1%, said Dr. Pratima Gupta, a board member for the American College of Obstetricians and Gynecologists.
“Any time a procedural abortion is done, the clinicians ensure that it was a complete abortion” by examining the tissue that is removed or performing an ultrasound during or after the procedure, she said.
Gupta, who has done abortion procedures for more than 20 years, said there are “very few complications from abortion — any kind of abortion, medication or procedural abortion.”
One study suggested that’s just as true for medication abortions that happen in a clinic, a doctor’s office or at home with the help of telehealth.
How does mifepristone’s safety and effectiveness compare to other drugs?
The FDA makes drug approval decisions on a case-by-case basis, weighing effectiveness, safety and other factors.
No drug is 100% effective, and many common medications don’t work for a significant portion of patients.
Antidepressants typically help between 40% and 60% of people with depression. New antibiotics approved by the FDA often resolve about 70% of infections.
Since 2000, roughly 6 million patients have taken mifepristone, according to the FDA. A 2021 review of agency records looking for deaths that were likely related to the drug identified 13, or 0.00027% of patients.
Medical organizations supporting mifepristone’s availability say the drug’s safety — given the rate of deaths — compares to “ibuprofen, which more than 30 million Americans take in any given day.” ___
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group and the Robert Wood Johnson Foundation. The AP is solely responsible for all content.

 Trump has begun another trade war. Here's a timeline of how we got here
Trump has begun another trade war. Here's a timeline of how we got here
 Canada's leader laments lost friendship with US in town that sheltered stranded Americans after 9/11
Canada's leader laments lost friendship with US in town that sheltered stranded Americans after 9/11
 Chinese EV giant BYD's fourth-quarter profit leaps 73%
Chinese EV giant BYD's fourth-quarter profit leaps 73%
 You're an American in another land? Prepare to talk about the why and how of Trump 2.0
You're an American in another land? Prepare to talk about the why and how of Trump 2.0
 Chalk talk: Star power, top teams and No. 5 seeds headline the women's March Madness Sweet 16
Chalk talk: Star power, top teams and No. 5 seeds headline the women's March Madness Sweet 16
 Purdue returns to Sweet 16 with 76-62 win over McNeese in March Madness
Purdue returns to Sweet 16 with 76-62 win over McNeese in March Madness