(Reuters) - Eli Lilly's Kisunla secured standard approval from the U.S. Food and Drug Administration on Tuesday for slowing the progression of Alzheimer's disease.
Kisunla is now the second drug to get a traditional approval from the U.S. regulator for the brain-wasting disease after Biogen and Japan-based Eisai's Leqembi, marking a major milestone in a field that has witnessed multiple failures.
The following is a list of companies with approved therapies in the U.S. market and those currently developing treatments that aim to modify the memory-robbing disease:

Company Drug or Stage of Mechanism
Therapy development &
indication
Eli Lilly

and Co Kisunla Secured U.S. FDA's Designed to
target and
standard approval remove toxic
for protein plaque
early Alzheimer’s in the brain
known as
beta-amyloid to
slow down
cognitive
decline
The An
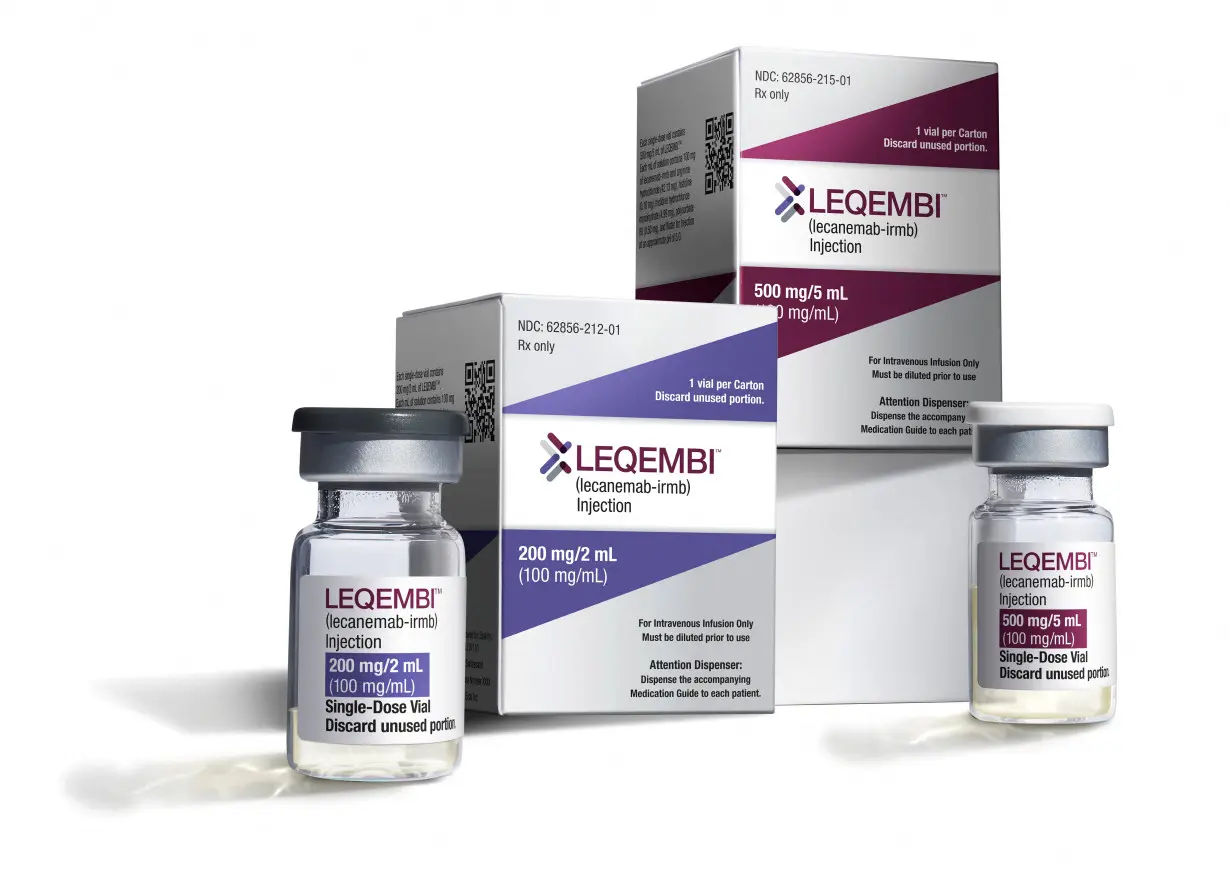
Eisai Co Leqembi first Alzheimer’s antibody
Ltd & treatment to designed to
Biogen Inc win remove sticky
beta-amyloid
standard approval deposits from
from the U.S. FDA the brain
BioVie NE3107 An oral small
Late-stage trial molecule that
for aims to inhibit
mild-to-moderate neuroinflammati
Alzheimer’s on and insulin
resistance
AB Science masitinib An oral
SA Late-stage study tyrosine kinase
for inhibitor that
mild-to-moderate targets immune
Alzheimer’s cells
responsible for
neuroinflammati
on
Annovis buntanetap An orally
Bio Mid-to-late-stage administered
trial for moderate small molecule
Alzheimer's inhibitor of
several
neurotoxic
proteins
Cognition CT1812 Oral drug
Therapeuti Mid-stage trial designed to
cs for bind to a
mild-to-moderate specific
Alzheimer’s receptor in
brain cells,
which mediates
beta-amyloid
attachment
Coya COYA 301 Designed to
Therapeuti Mid-stage trial inhibit
cs for molecules
mild-to-moderate responsible for
Alzheimer’s neuroinflammati
on and improve
cognitive
function
Actinogen xanamem Blocks the
Medical Mid-stage trial production of
Limited for the stress
mild-to-moderate hormone
Alzheimer's cortisol,
associated with
cognitive
impairment, in
brain cells
AC Immune ACI-24.060 An
SA Early-to-mid-stage antibody-based
trial for immunotherapy
Alzheimer’s vaccine
designed to
target
beta-amyloid
plaque
Biogen BIIB080 An antisense
Mid-stage trial oligonucleotide
for early-stage therapy
Alzheimer’s directed
against "tau"
proteins
Longeveron lomecel-B A cell therapy
Mid-stage trial designed to
for mild stimulate
Alzheimer’s neuroregenerati
on and prevent
the progression
of the disease
(Reporting by Pratik Jain and Mariam Sunny in Bengaluru; Editing by Maju Samuel and Pooja Desai)

 Bird flu spreads to mammals, fears of human transmission
Bird flu spreads to mammals, fears of human transmission
 US Supreme Court turns away casino mogul Wynn's bid to challenge NY Times v. Sullivan defamation rule
US Supreme Court turns away casino mogul Wynn's bid to challenge NY Times v. Sullivan defamation rule
 Brazil to pursue 2026 primary surplus target despite challenges, minister says
Brazil to pursue 2026 primary surplus target despite challenges, minister says
 Venezuela receives hundreds of deported migrants from US after flights restart
Venezuela receives hundreds of deported migrants from US after flights restart
 US business activity rises in March, but sentiment deteriorates further
US business activity rises in March, but sentiment deteriorates further
 Japan's cherry blossom season begins as first blooms appear in Tokyo
Japan's cherry blossom season begins as first blooms appear in Tokyo
 Iowa hires McCollum, who swept Missouri Valley titles and won NCAA game in his one season at Drake
Iowa hires McCollum, who swept Missouri Valley titles and won NCAA game in his one season at Drake