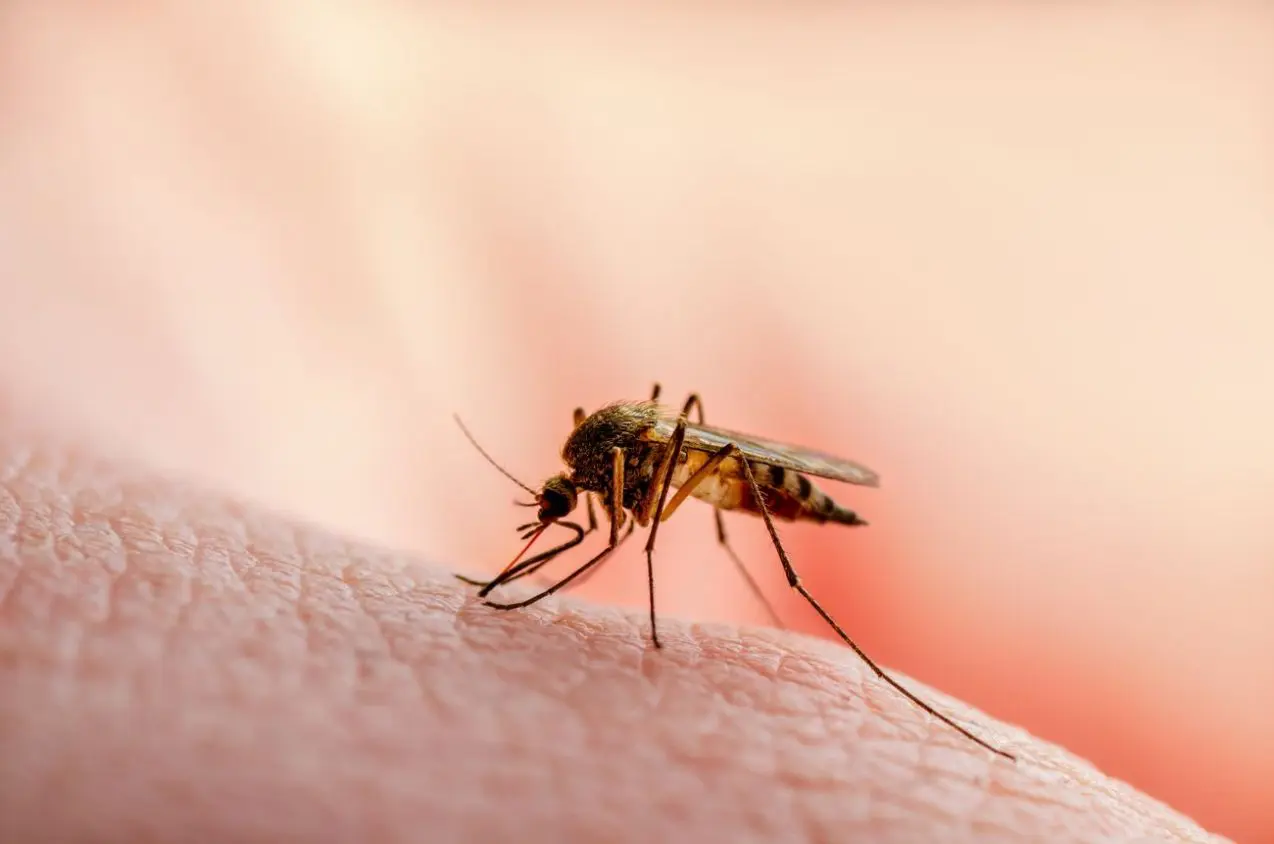
In an ambitious global experiment, scientists are unleashing millions of engineered mosquitoes carrying virus-blocking bacteria upon dense urban landscapes as an ecological solution to infectious diseases transmitted by the insects’ bites. Initial trials suppressing dengue spread by up to 80 percent bolster hopes of harnessing evolution to vanquish humankind’s most lethal animal foe, responsible for nearly a million annual deaths. But realizing lasting impacts across continents presents daunting financial and logistical hurdles.
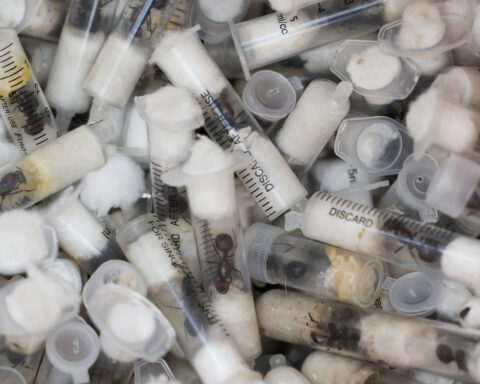
At dedicated factories in Colombia, technicians mass-produce Aedes aegypti mosquitoes infected with the Wolbachia parasite, which largely prevents transmission of viruses like dengue. The ambitious aim: completely replace normal mosquito populations with harmless counterparts passing down lifelong infection. By swapping a few microbes, communities escaping seasonal scourges glimpse improved health, lowered suffering and medical savings.
The audacious plan’s successes stem from observing Wolbachia’s blocking abilities in wild mosquitoes, while tackling the challenge of efficiently distributing it to dangerous urban-dwelling species. Through meticulous lab work spanning decades, scientists injected the bacteria into eggs of targeted insects. Then trials monitored infection flourishing after releasing manipulated mosquitoes to outcompete normal counterparts for mates. Today custom-designed drones and community partnerships facilitate continual blanket coverage mimicking vaccine campaigns.
But logistical obstacles abound, demanding extensive personnel and technology investments over years before communities experience benefits. And while initial statistics seem promising, inconsistent establishment success and incomplete picture of incidence fluctuations fuel expert debate over ideal roll-out strategies. With diseases expanding ranges amid climate shifts, the vision of deploying biotechnological shields globally further strains limited budgets. Conflicting opinions linger on managing inevitable pathogen evolution over time as well.
Still, realized even partially, the transformative potential seems immense given mosquitoes’ incredibly vast harmful impacts on global health and economies. Dengue alone already sickens 400 million annually, killing over 20,000, with outbreak severity spiraling in epidemics overwhelming hospitals. And Aedes’ climate change-assisted migration ensures more populations will battle the brutal infection nicknamed “breakbone fever.” Its prevention could thus reverberate widely in lowering suffering, saving lives and boosting financial outcomes when broadly instituted.
Indeed, for afflicted locales, something as conceptually simple as altered mosquito physiology promises liberation from dreadful infectious disease seasons haunting community memory. With insecticides faltering against tenacious bugs, this biotechnical end-run neatly sidesteps resistance issues via evolutionary craftiness. And the infectious agent itself enduringly protects future generations instead of demanding endless respraying. As one previous dengue patient put it, forgetting about the illness altogether would bring tremendous progress.
So aside from surmounting hurdles in finance and production, the lasting question remains if viruses eventually evolve workarounds. But even if Wolbachia only buys vulnerable communities a few decades reprieve, scientists consider that radically game-changing. Short generational timescales mean mosquitoes and microbes operate on vastly accelerated biological clocks suited to rapid adaptation solutions. Buying time allows better preparations for when pathogens overcome hardy bacteria passed down mosquito lineages. With a large enough head start, human innovation could then be poised to respond via next-stage interventions like targeted gene editing.
Thus fleeting successes on evolutionary time horizons nonetheless promise dramatic gains for societies beleaguered by mosquitoes today, especially when contemplating promising complementary solutions. Wolbachia surges end current seasonal spikes in illnesses like dengue essentially overnight. And parallel research edges towards unleashing similar tactics against malaria-transmitting mosquitoes haunting African regions also plagued by dengue flare-ups. With incremental breakthroughs stacking, a future looks imaginable where multiplescourges afflicting millions annually fade against shrewd counter-adaptations. Even imperfect and temporary, coordinated biotechnical strikes offer glimpses of profound progress.

 Trump has begun another trade war. Here's a timeline of how we got here
Trump has begun another trade war. Here's a timeline of how we got here
 Canada's leader laments lost friendship with US in town that sheltered stranded Americans after 9/11
Canada's leader laments lost friendship with US in town that sheltered stranded Americans after 9/11
 Chinese EV giant BYD's fourth-quarter profit leaps 73%
Chinese EV giant BYD's fourth-quarter profit leaps 73%
 You're an American in another land? Prepare to talk about the why and how of Trump 2.0
You're an American in another land? Prepare to talk about the why and how of Trump 2.0
 Chalk talk: Star power, top teams and No. 5 seeds headline the women's March Madness Sweet 16
Chalk talk: Star power, top teams and No. 5 seeds headline the women's March Madness Sweet 16
 Purdue returns to Sweet 16 with 76-62 win over McNeese in March Madness
Purdue returns to Sweet 16 with 76-62 win over McNeese in March Madness