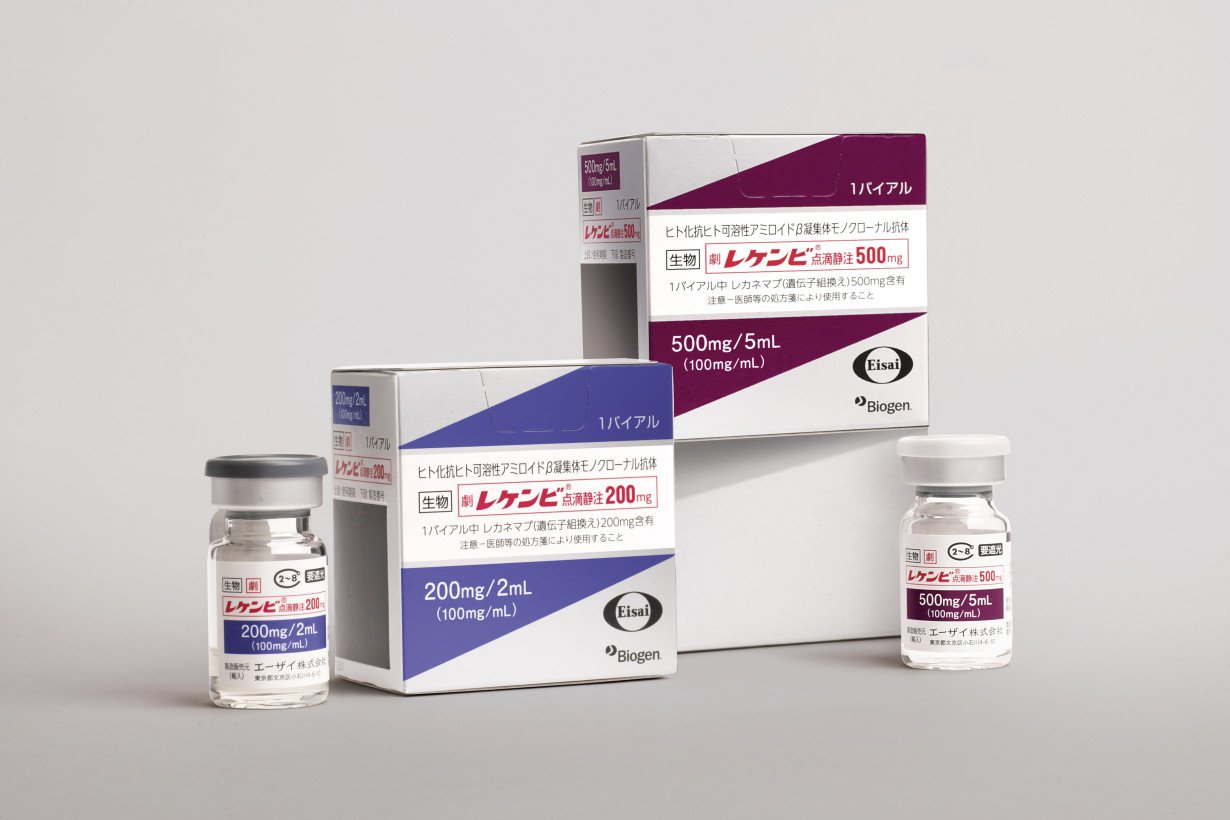
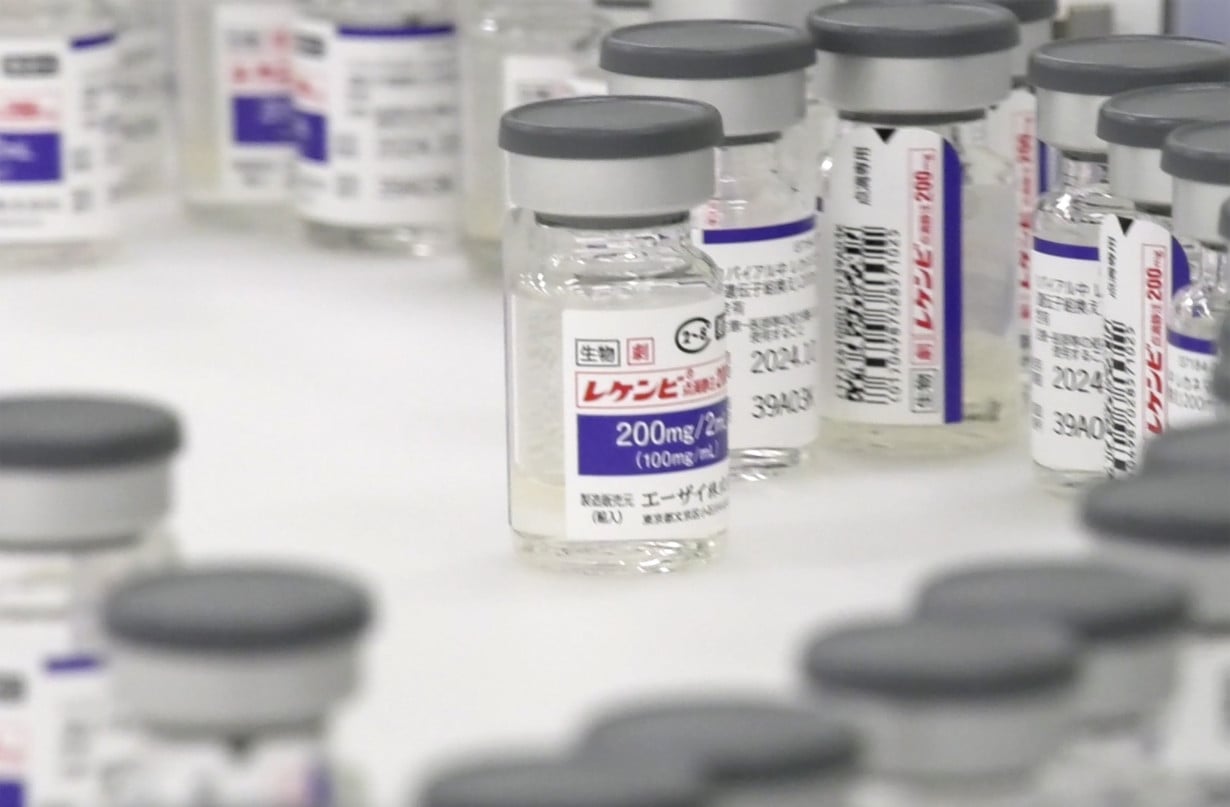
TOKYO (AP) — Japan's health ministry has approved Leqembi, a drug for Alzheimer’s disease that was jointly developed by Japanese and U.S. pharmaceutical companies. It's the first drug for treatment of the disease in a country with a rapidly aging population.
Developed by Japanese drugmaker Eisai Co. and U.S. biotechnology firm Biogen Inc., the drug's approval in Japan comes two months after it was endorsed by the U.S. Food and Drug Administration.
Leqembi is for patients with mild dementia and other symptoms in the early stages of Alzheimer's disease, and the first medicine that can modestly slow their cognitive decline.
Prime Minister Fumio Kishida, who announced Japan's approval of Leqembi on Monday, called it “a breakthrough” and said that the “treatment of dementia has now entered a new era.”
Kishida has pledged to step up support for the growing number of dementia patients and their families and is due to launch a panel this week to discuss measures for a dementia-friendly society.
According to the health ministry, Japan's number of dementia patients who are 65 years of age or older will rise to 7 million in 2025, from the current 6 million.
The drug, however, does not work for everyone and — as with other Alzheimer's drugs that target plaques in the brain — can cause dangerous side effects such as brain swelling and bleeding in rare cases.
Eisai said it will conduct a post-marketing special use survey in all patients administered the drug until enough data is collected from unspecified number of patients under Japanese health ministry procedures.
The drug will be partially covered by health insurance and is expected to be ready for clinical use by the end of the year. The price is yet to be decided but is expected to be expensive, Kyodo News agency reported.
Eisai is committed to delivering Leqembi to people who need it and their families “as a new treatment,” said Haruo Naito, the company’s CEO.
“We aim to create impact on issues surrounding dementia in Japanese society," he said.

 Central bank frenzy meets political storm
Central bank frenzy meets political storm
 Boeing, Apple, GE to join US business mission to Vietnam next week, list shows
Boeing, Apple, GE to join US business mission to Vietnam next week, list shows
 Stellantis to supply Italy's Iveco with two EV vans for Europe
Stellantis to supply Italy's Iveco with two EV vans for Europe
 As Trump thaws ties, Russia has a new public enemy number one: Britain
As Trump thaws ties, Russia has a new public enemy number one: Britain
 WHY THE GOV (PROBABLY) WON'T SHUT DOWN TONIGHT (4aET)
WHY THE GOV (PROBABLY) WON'T SHUT DOWN TONIGHT (4aET)
 Shohei Ohtani and four other Japanese players come home to start the MLB season
Shohei Ohtani and four other Japanese players come home to start the MLB season
 Millions of people celebrate Holi, the Hindu festival of colors
Millions of people celebrate Holi, the Hindu festival of colors
 Olympics-Olympians make climate plea to IOC presidential candidates
Olympics-Olympians make climate plea to IOC presidential candidates